Organic Chemistry Section
Department of Inorganic and Organic Chemistry
Research
Research Lines
People
Links
- Catalysis
Stereoselective formation of carbon-carbon bonds on the basis of enolates generated catalytically with nickel(II) complexes
We are interested in developing new asymmetric synthesis methods based on the activation of structurally simple and easily manipulable metal complexes in the reaction medium. The resulting species can catalyze the formation of enolates from N-acyl thioimides, for subsequent use in the stereoselective construction of carbon-carbon bonds. The reactions involved are thus direct reactions based on N-acyl thioimides.
This approach's credibility was confirmed in studies on the addition of N-acyl-4-isopropyl-1,3-thiazolidine-2-thiones to methyl orthoformate or diarylmethyl methyl ethers in the presence of substoichiometric quantities of simple, commercially accessible nickel(II) complexes, (R3P)2NiCl2.
The reaction between these complexes and TESOTf notably increases the metal's electrophilicity, facilitating the enolization of the N-acyl thioimides. At the same time, the TESOTf turns the electrophiles into intermediates that react with the nickel(II) enolates on the basis of an SN1 mechanism. Control over the configuration of the stereocenter at C-α is excellent.
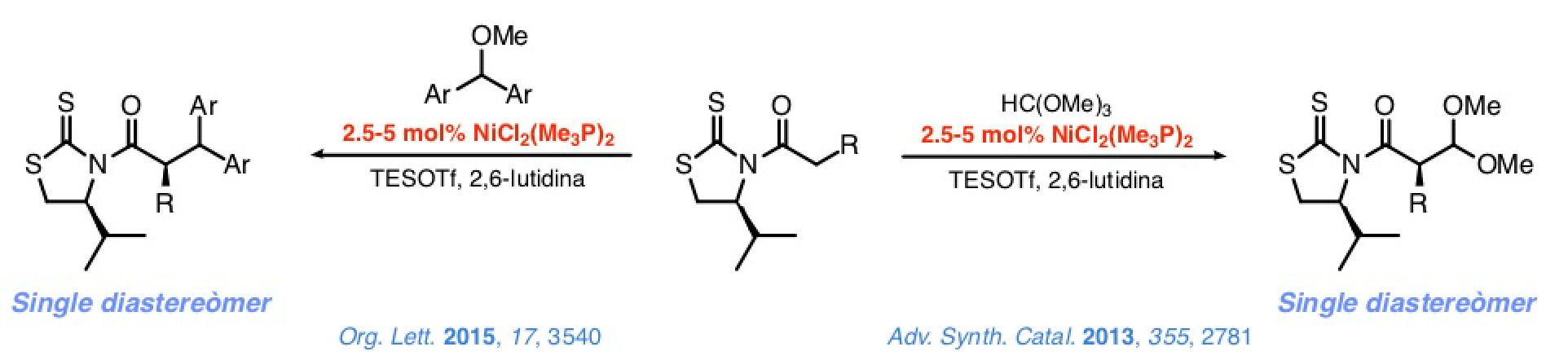
Similar control over the configuration of the stereocenter at the β-position is also good but in general somehow poorer. Nevertheless, addition of the N-azidoacetyl thioimide to dialkyl acetals from aromatic or cobalt-protected propargylics aldehydes leads to the corresponding 2,3-anti adducts with an excellent stereocontrol. A proof of the synthetic potential of such a method is the synthesis of tripeptides containing a β-hydroxytyrosine moiety at the central position, which can be completed satisfactorily in a few steps and with a high efficiency.
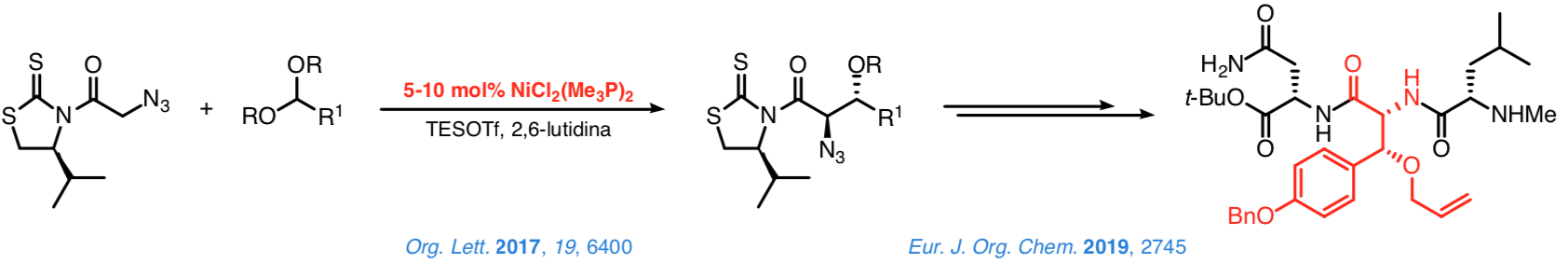
Taking advantage of the former reactions, we are especially interested in developing a general approach based on the use of chiral catalysts that enable us to attain a highly effective stereocontrol over the configuration of the new stereocenters. In this context, we have recently established that the N-acyl-1,3-tiazinan-2-thiones are suitable platforms from which to carry out direct and enantioselective reactions catalyzed by [DTBM-SEGPHOS]NiCl2.
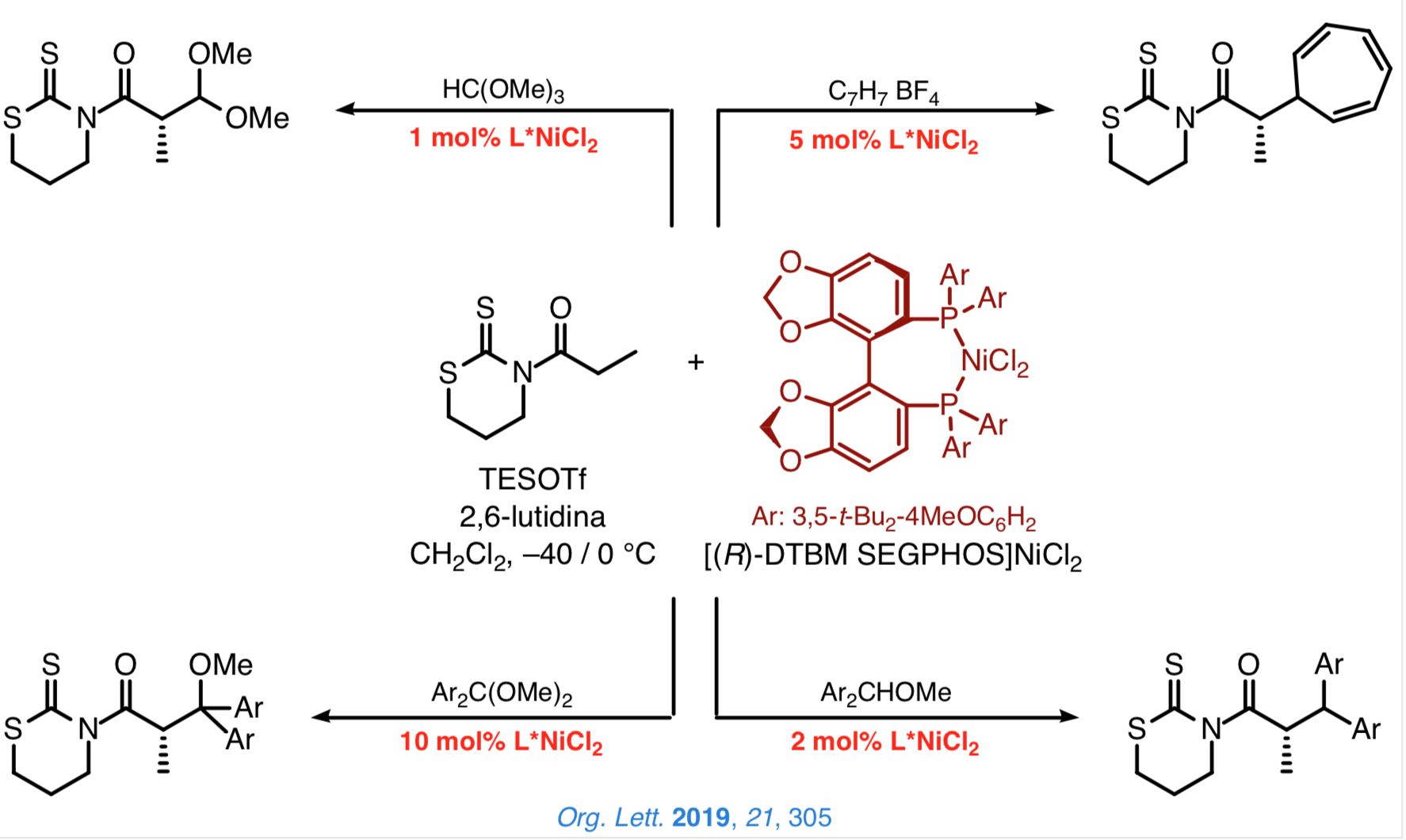
We are currently exploring new processes for the direct and enantioselective construction of carbon–carbon bonds catalyzed by chiral nickel(II) complexes that involve the simultaneous installation of two new stereocenters.
- Titanium enolates as biradicals
Stereoselective formation of carbon-carbon and carbon-oxygen bonds through reaction with radicals
|
The discovery of the biradical character of titanium(IV) enolates from α-hydroxy ketones a few years ago resulted in the emergence of a new field of study aimed at understanding this surprising characteristic and taking advantage of the new radical reactivity stemming from it. Once established that the biradical nature of such enolates hinges on a valence tautomery, whose origin lies on changes of the Ti–O distance, we intend to develop new methods for the stereoselective construction of carbon–carbon and carbon–oxygen bonds from chiral titanium(IV) enolates and radicals. |
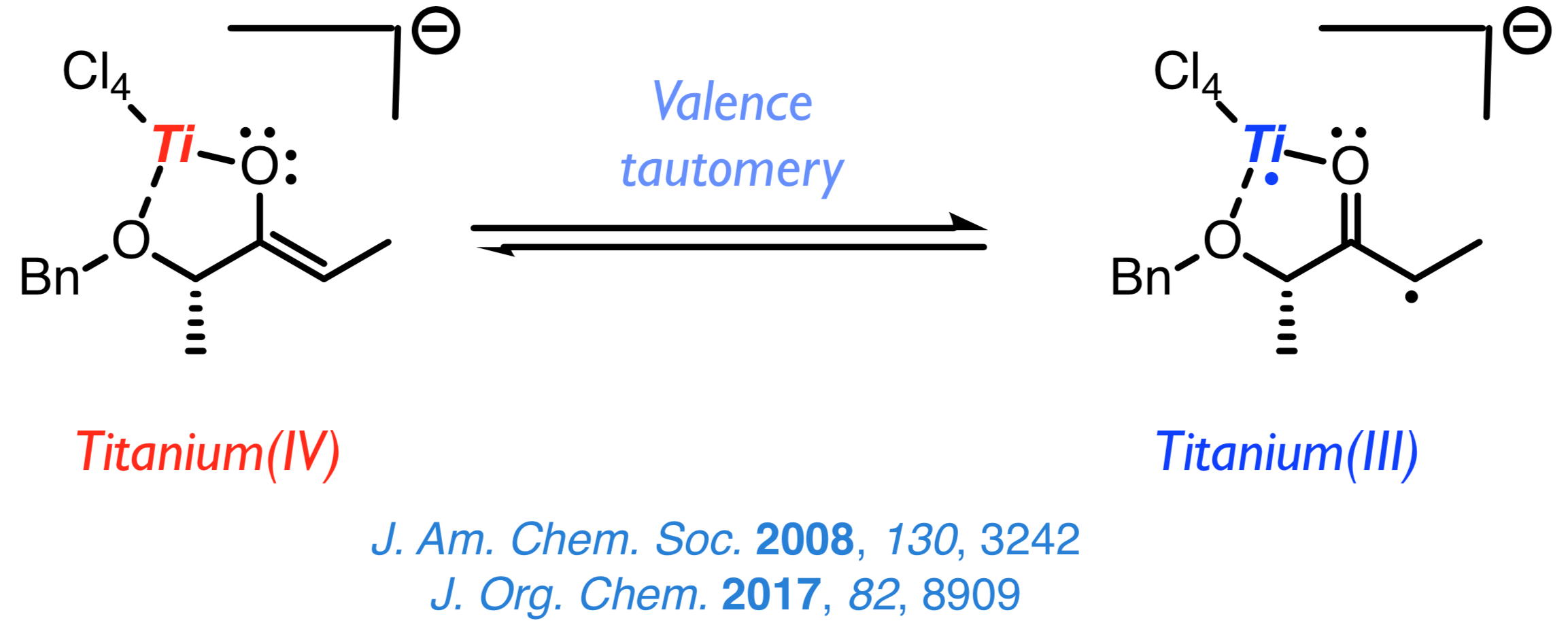
|
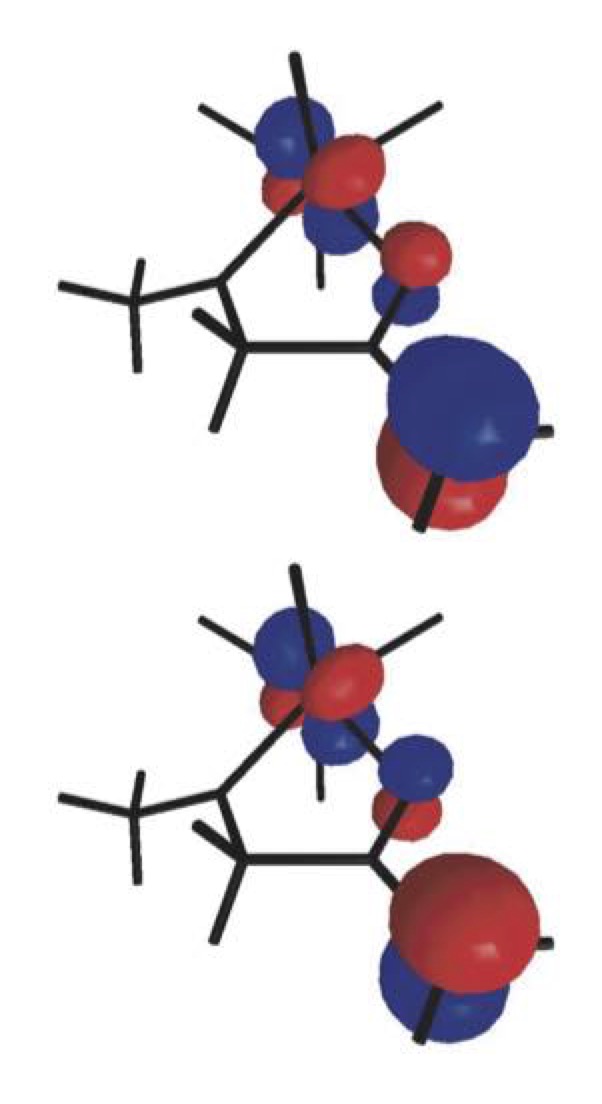
|
Comprehensive studies on the most suitable scaffolds for such transformations have shown that both chiral oxazolidinones and oxazolidinethiones are excellent platforms from which to address the construction of carbon–oxygen bonds using TEMPO and molecular oxygen without the need for any other reagent as represented in the next scheme.
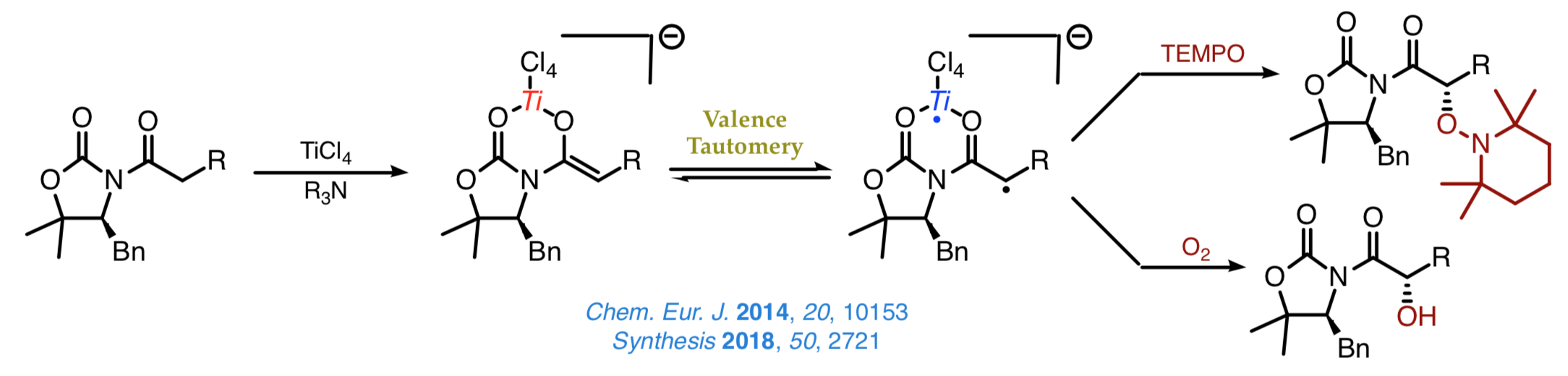
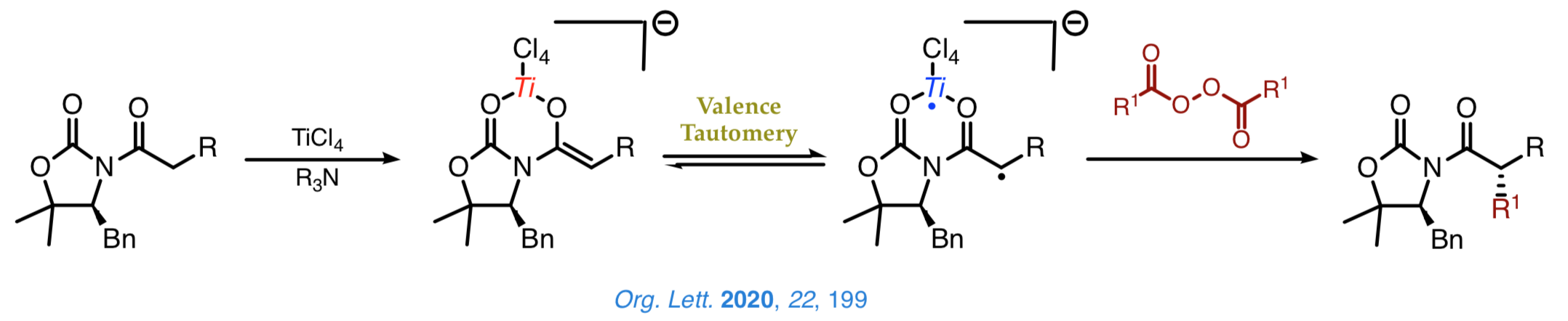
We are current examining new radical-like approaches to the stereoselective construction of carbon–carbon bonds that enable us the introduction of a range of bulky groups, which cannot be easily achieved by other synthetic methods. A nice example involves the decarboxylative alkylation shown below, which proceeds with a high chemoselectivity and an outstanding control over the configuration of the Cα stereocenter.
- Titanium enolates as nucleophiles
Stereoselective formation of carbon-carbon bonds: aldol and Michael additions
In recent years, chiral ketones have become a highly useful tool for the substrate-controlled stereoselective construction of carbon-carbon bonds. In particular, our group has put a great deal of work into studying the aldol reactions of titanium enolates from α- and β-hydroxy ketones. In the case of the former, we have established the vital role that titanium Lewis acids and protecting groups play in the configuration of the resulting aldol. With regard to the latter, we have developed methods through which excellent results can be obtained in the aldol reactions of methyl, ethyl and isopropyl ketones derived from Roche ester.

|
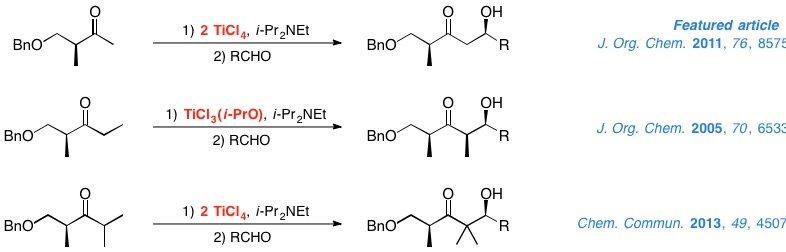
|
We are currently studying aldol reactions for the stereoselective construction of quaternary centers and Michael additions to enones and nitroalkenes.
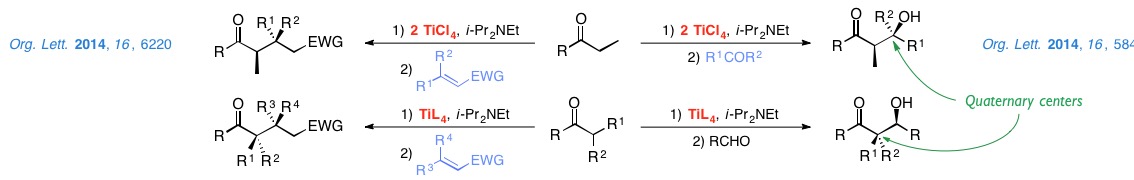
- • Synthesis of Natural Products
Application of the aforementioned methodologies to the synthesis of natural products
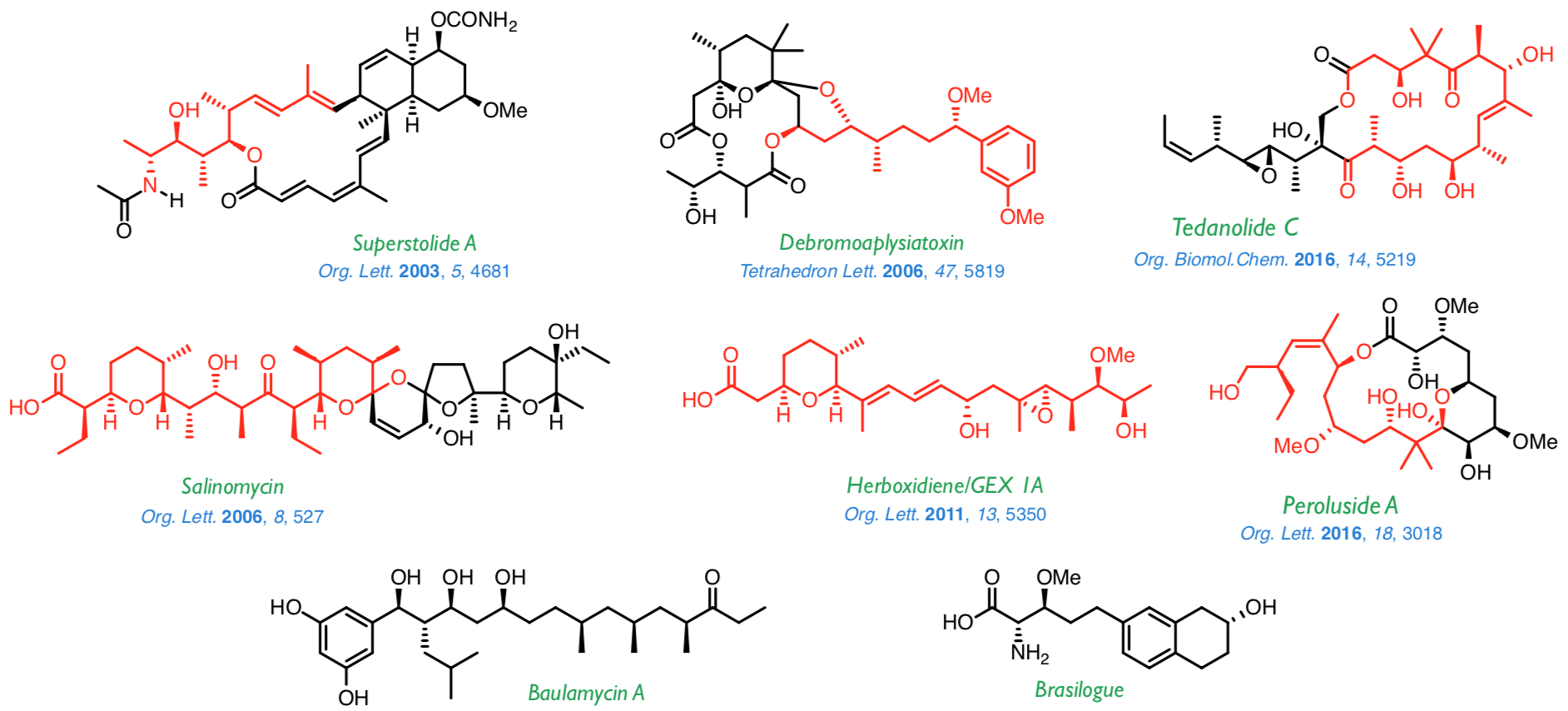
The methodologies described above have been successfully applied to the synthesis of a wide range of natural products, such as those shown below. At present, we are working on the synthesis of baulamycin A and brasilogue.